In our research we strive to achieve synthetic control over functional nanostructures and to investigate their physical properties and their application as materials for energy conversion and storage. We try to accomplish this by means of manifold synthetic approaches ranging from wet chemistry to chemical and physical deposition. To deepen our understanding of the working principles of materials and devices we employ a diverse line-up of analytical techniques to study important characteristics such as morphology, electrochemical and optoelectronic properties and structure-function relations.
The structure of our research is mainly divided in four different subgroups: Covalent-Organic and Metal-Organic Frameworks, Nanomorphologies, Photovoltaics and Mesoporous Nanoparticles for Biomedical Application.



NEWS
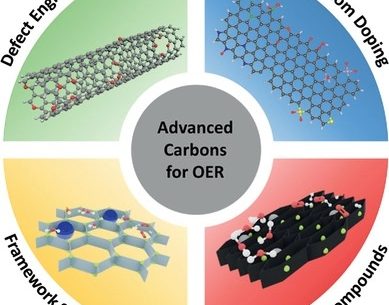 Carbon-based materials against the carbon footprint: the future of energy storage by water electrolysis
Carbon-based materials against the carbon footprint: the future of energy storage by water electrolysis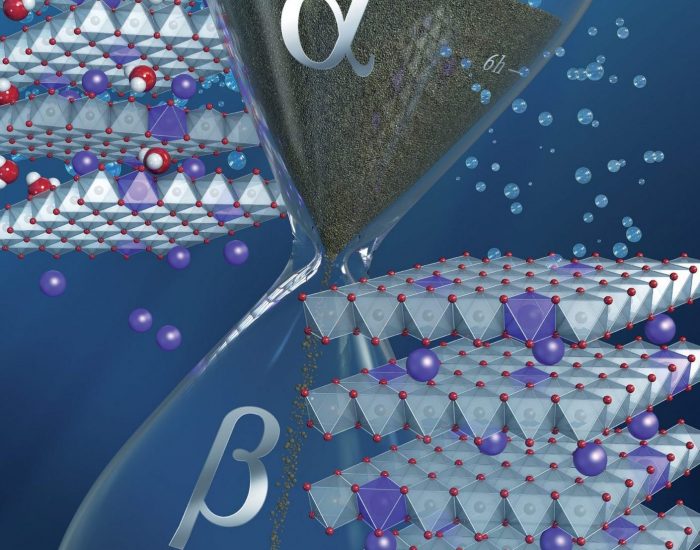 V is for voltage: V(III)-doped nickel oxide based nanocatalysts, in depth:
V is for voltage: V(III)-doped nickel oxide based nanocatalysts, in depth: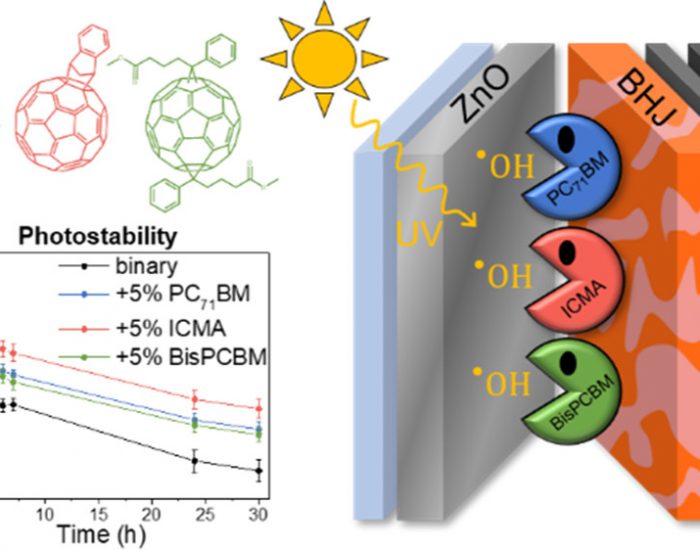 All good things come in threes: how Fullerene Derivative Additives drastically improve the stability of inverted ternary organic solar cells
All good things come in threes: how Fullerene Derivative Additives drastically improve the stability of inverted ternary organic solar cells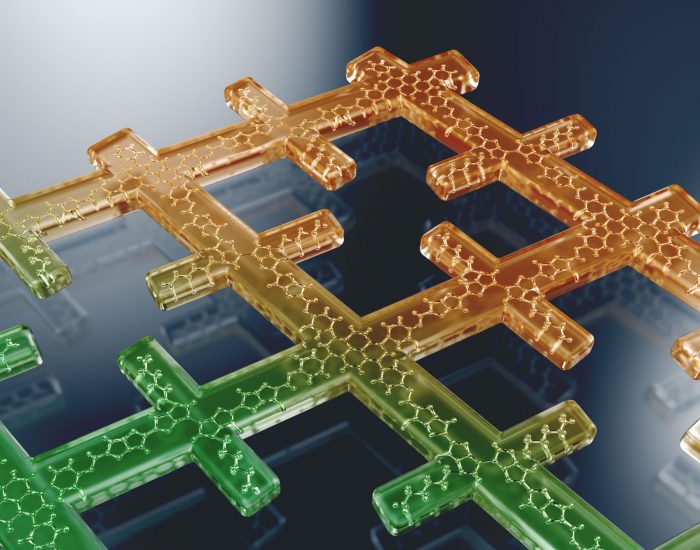 Fast electrochromism in covalent organic frameworks: highly promising coatings for smart glass
Fast electrochromism in covalent organic frameworks: highly promising coatings for smart glass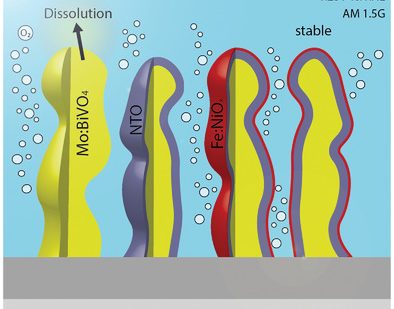 Protect Ya Film: splitting water longer with ultra-thin coated Mo:BiVO4
Protect Ya Film: splitting water longer with ultra-thin coated Mo:BiVO4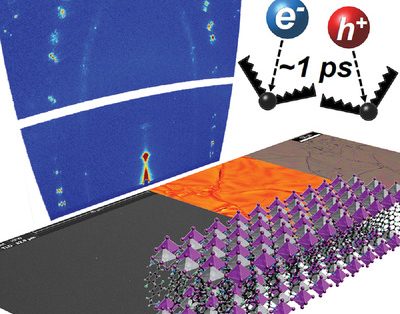 No need for GPS: Ultrafast carrier localizing in highly oriented thin films
No need for GPS: Ultrafast carrier localizing in highly oriented thin films Message in a bottleneck: the performance limiting factors in lead-free perovskite solar cells
Message in a bottleneck: the performance limiting factors in lead-free perovskite solar cells
Research topics in the group
Images by Christoph Hohmann