Selected Publications
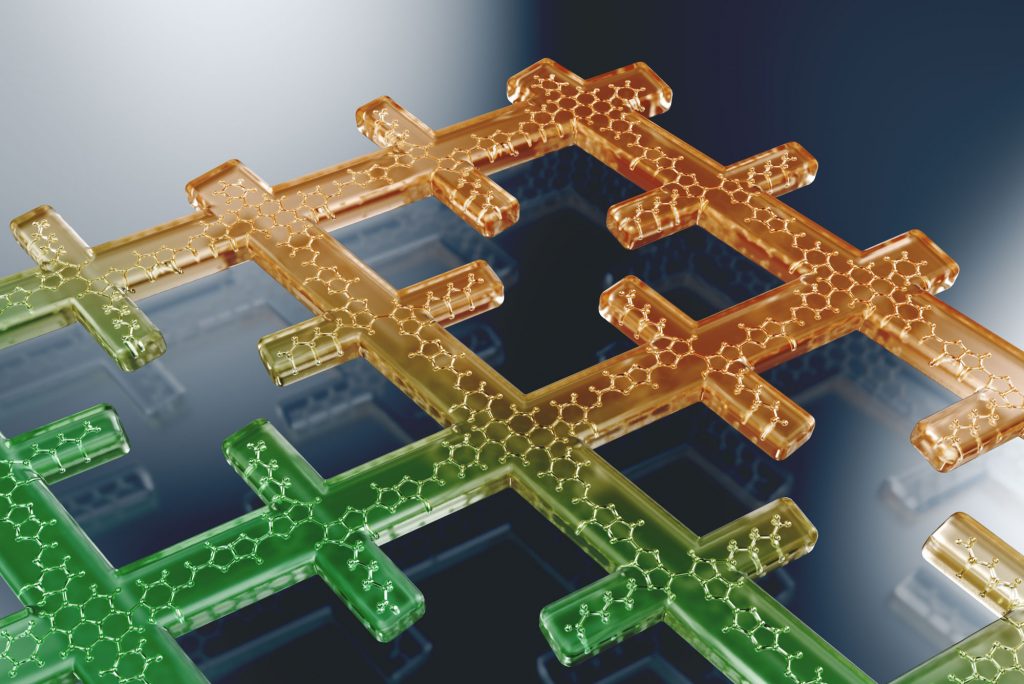
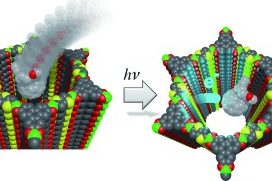
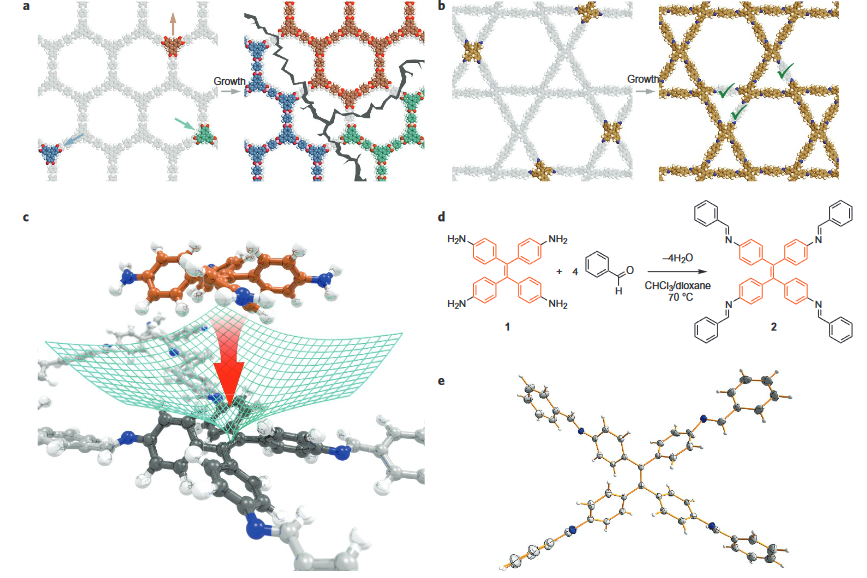
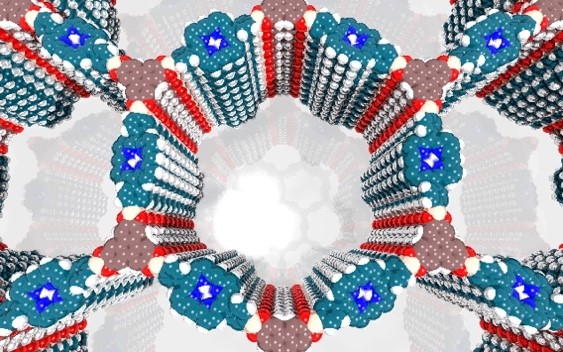
Covalent organic frameworks (COFs) represent a fascinating class of crystalline porous materials obtained by reversible condensation reactions of versatile organic building blocks. The ability to control the structure of the resulting COFs through the symmetry, structure and linkage chemistry of their building blocks creates numerous possibilities for the synthesis of novel crystalline framework materials. These synthetic endeavors result in two-dimensional polymers that stack in the third dimension via dispersive interactions (2D COFs), or in covalently-linked three-dimensional crystalline polymers (3D COFs). In addition, numerous molecular functionalities can be embedded into these frameworks by employing functionalized building blocks. These construction concepts greatly enhance our ability to design porous organic materials with predictable structures, desired properties and functionalities such as adsorption, luminescence, (photo)catalysis, or chemical sensing. See Fig. 1 for an example of a stacked 2D COF structure-
Following these design principles, photoactive molecular building blocks such as porphyrins or heteroaromatic chromophores can be spatially integrated into the backbone of COFs, allowing for the construction of crystalline model systems for organic bulk heterojunctions, chemical sensors and porous electrodes for photoelectrochemical systems. This includes means of controlling the morphology and packing order of COFs in thin films [1] and with spatially locked-in building blocks.[2]
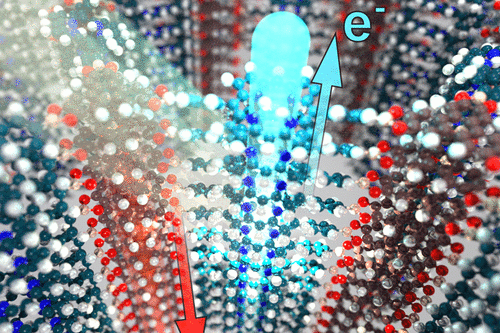
We have developed different strategies aimed at creating electroactive networks capable of light-induced charge transfer. For example, we have developed a COF containing stacked thienothiophene-based building blocks acting as an electron donor constituent with a 3 nm open pore system, which show light-induced charge transfer to an intercalated fullerene acceptor phase.[3] Adding to the guest acceptor inclusion paradigm, we have designed a COF integrated heterojunction consisting of alternating columns of stacked donor and acceptor molecules, promoting the photo-induced generation of charge carriers inside the COF network.[4] Furthermore, synthetic efforts have led to several COFs with strong optical absorption integrating extended chromophores capable of efficient harvesting of visible and near infrared light. [5, 6].
Extending newly developed thin film growth methodology to a solvent-stable oriented 2D COF photoabsorber structure, we have established the capability of COF films to serve in photoelectrochemical water splitting systems.[7] The detailed mechanism of excited state dynamics in light-harvesting conjugated COFs has been revealed by means of transient absorption spectroscopy.[8] Many optoelectronic applications of COFs depend on significant electrical conductivity. Here, Wurster-type structural motifs are attractive building blocks for imparting high conductivity in the corresponding COFs.[9] COF films can also act as ultrafast solvatochromic chemical sensors [10] and show very efficient and fully reversible electrochromic response with fast color switching times.[11]
We are presently extending novel concepts towards the design of COFs comprising built-in heterojunctions with ultralarge channels, redox-active and ion-conducting COFs, as well as COFs featuring strong electronic coupling in three dimensions.
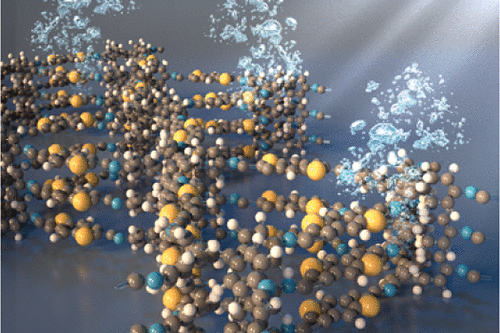
Metal-organic frameworks (MOFs) are crystalline porous frameworks formed by a bottom-up approach from molecular building blocks with a distinct geometry and metal-based nodes. The synthesis of MOFs is highly modular and therefore their pore size, pore shape, dimensionality of the pore system and the chemical environment in the pores can be controlled by the judicious selection of their building blocks in combination with their mode of assembly. Based on this powerful structural paradigm, we develop model systems with periodic, (interpenetrating) networks of electron donor- and acceptor-phases. Here, we invoke the concepts of reticular chemistry to create two- and three-dimensional MOFs with significant electrical conductivity and diverse optoelectronic properties namely by control over the relative spatial orientation of their molecular building blocks, their wall-thickness, and their overall orientation relative to a substrate. In collaboration with theoreticians, we aim at enhancing our understanding of the relationship between the electronic and structural parameters and the emerging (opto)electronic behavior.
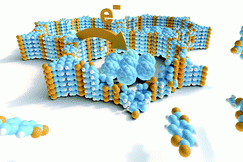
Recent work has been directed towards the electrically conducting two-dimensional (2D) M-CAT-1 MOF series and MOF-74 topologies in the form of bulk and thin film systems. Here, we established vapour-assisted conversion film synthesis for the M-CAT-1 family. Notably, photo-induced charge-carrier generation and extraction could be identified, thus establishing M-CAT-1 as photo-active materials. New members of both the M-CAT-1 family and the MOF-74 topology were introduced by employing different metal-ions and expanding the variety of suitable organic ligands, respectively. For example, electrical conductivity enhanced by several orders of magnitude was discovered for the zinc analog of the expanded MOF-74 structure, and theoretical calculations suggest specific pathways for the favorable charge carrier trajectories in these intriguing materials. Further work is, for example, directed towards controlling the dispersive interactions of expanded structural linkers with a view on increasing electronic communication for charge carriers in these networks.
REFERENCES
- Medina et al., J. Am. Chem. Soc. 2015, 137, 1016.
- Ascherl et al., Nature Chem. 2016, 8, 310.
- Dogru et al., Angew. Chem. Int. Ed. 2013, 52, 2920.
- Calik et al., J. Am. Chem. Soc. 2014, 136, 17802.
- Keller et al., J. Am. Chem. Soc. 2017, 139, 8194.
- Bessinger et al., J. Am. Chem. Soc. 2017, 139, 12035.
- Sick et al., J. Am. Chem. Soc. 2018, 140, 2085.
- Jakowetz et al., J. Am. Chem. Soc. 2019, 141, 11565.
- Rotter et al., Chem. Sci. 2020, 11, 12843.
- Ascherl et al., Nature Commun. 2018, 9, 3802.
- Bessinger et al., J. Am. Chem. Soc. 2021, 143, 7351.