Selected Publications
Controlling Cellular Function with Multifunctional Porous Nanoparticles
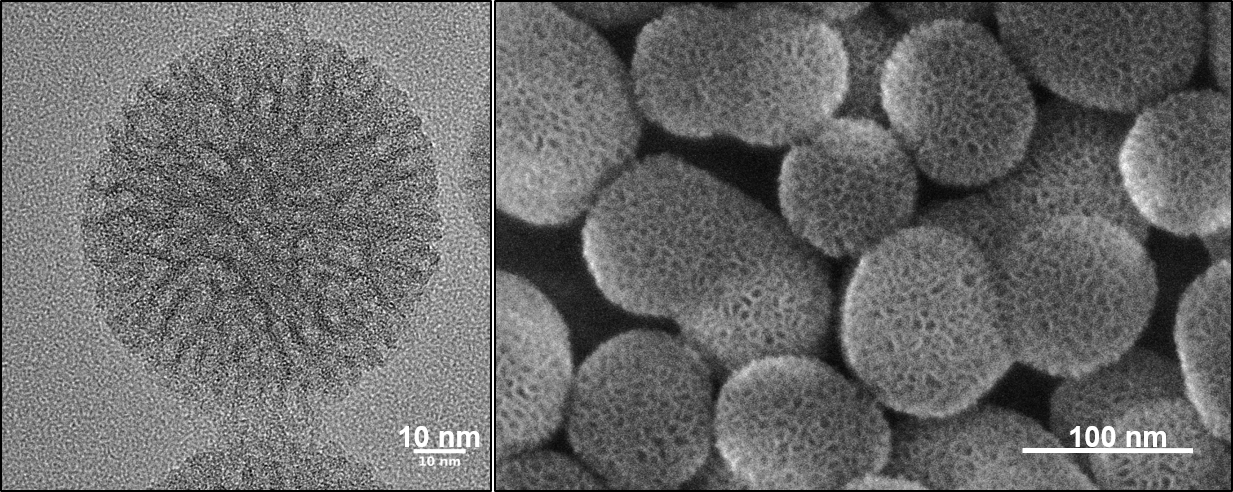
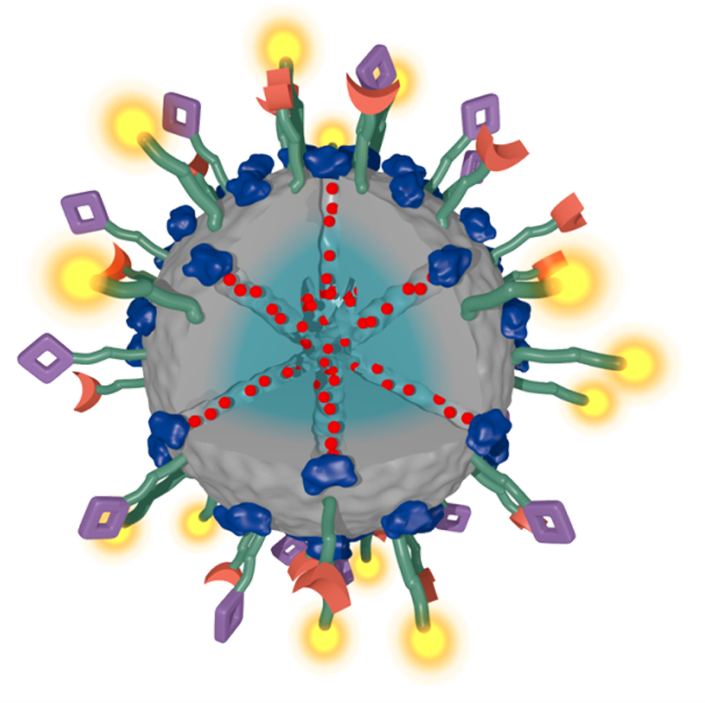
Over the years, numerous intriguing types of multifunctional mesoporous nanoparticles have been developed in our group as carriers for targeted drug delivery, for example in the context of cancer therapy. In our recent work, we have addressed several key challenges related to the development of multifunctional mesoporous silica and other mesoporous nanoparticles, including stimuli-responsive release systems, targeting ligands for specific cellular receptors, biodistribution and biocompatibility.
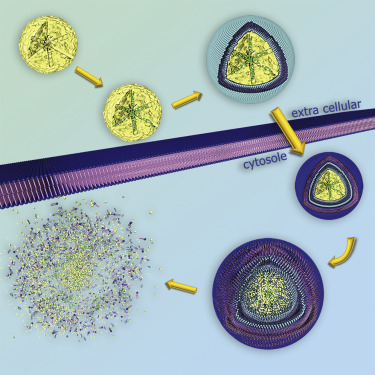
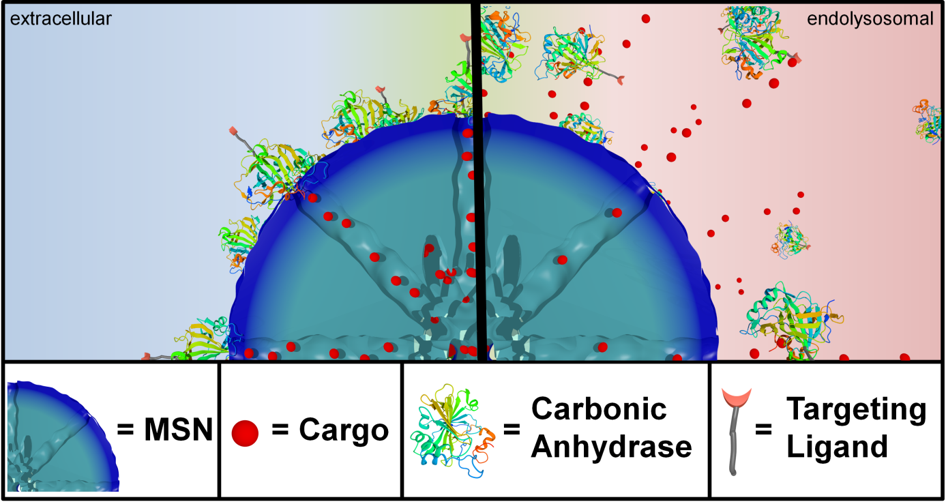
For example, folate and epidermal growth factor (EGF) have been used for successful cell targeting with mesoporous silica nanoparticles (MSNs). Considering triggered release in the endosome, a novel pH-responsive system has been created based on genetically modified carbonic anhydrase (CA) gatekeepers. A pH-dependent CA inhibitor was covalently attached to the surface of the MSNs, resulting in the desired opening mechanism caused by the endosomal pH change. Addressing endosomal escape, we have covalently attached a red-light sensitive phthalocyanine photosensitizer to the MSN, surrounded by a lipid bilayer, which releases cargo upon illumination. Moreover, we have exploited the proton sponge effect during acidification of the endosome with polymer-functionalized MSNs to achieve endosomal release. Taking advantage of overexpressed matrix metalloproteinases in cancer tissue, we have also achieved spatially selective extracellular release of bioactive molecules.
Organically functionalized MSNs were developed to reversibly adsorb oligonucleotides such as siRNA at high loading and with high cellular activity upon release. Moreover, cellular uptake of proteins such as small antibody fragments was successfully achieved using a novel release mechanism from MSNs.
An important feature of the MSNs regards their biodistribution and their fate after successful drug delivery. Our recent quantitative analysis of the dissolution kinetics of different types of multifunctional MSNs demonstrates that their dissolution rate can be tuned over a wide range, leading to lifetimes of under one hour to several weeks in aqueous media. In contrast, these MSNs are stable in ethanol for long periods exceeding two years.
Recently, we have expanded the scope of mesoporous materials to mesoporous metal phosphates with very high surface area and the ability to dissolve in the acidic cytosol. Their intracellular behavior opens new vistas in targeted drug release with porous nanoparticles. Notably, our novel amorphous mesoporous calcium phosphate-citrate nanoparticles (CPCs) with an average size of 50 nm act intrinsically as anti-cancer agents. Internalized CPCs dissolve in the acidic endosomes, rapidly releasing calcium ions and citrate into the cytosol, thus inducing the desired cell death (apoptosis) of cancer cells. Remarkably, the cytotoxicity of CPCs is cell-specific. Mesenchymal cancer cell lines are much more strongly affected by the calcium/citrate shock and induce apoptosis at lower IC50 values than epithelial cancer cells, while healthy cells are not affected. These nanoparticles show promising therapeutic activity in a malignant pleural effusion (MPE) mouse model. Our results strongly suggest the possibility to efficiently treat certain lung tumors while at the same time dramatically reducing toxic side effects.
Purely organic nanoparticles featuring molecule-sized cavities can be created by covalently crosslinking cyclodextrins with spacer molecules to form drug carriers with high loading capacity (CD-NP). The nanoparticles are biocompatible, highly water-dispersible, and of a size of about 150 nm, rendering them well suited for theranostic applications.
Fast sugar-mediated cell-uptake kinetics was observed on HeLa cells, revealing particle uptake within less than half an hour. The nanoparticles could be loaded with different cargo molecules showing pH-responsive release behavior. Hence, effective cell killing with doxorubicin as cargo molecules was shown in live-cell experiments, respectively.
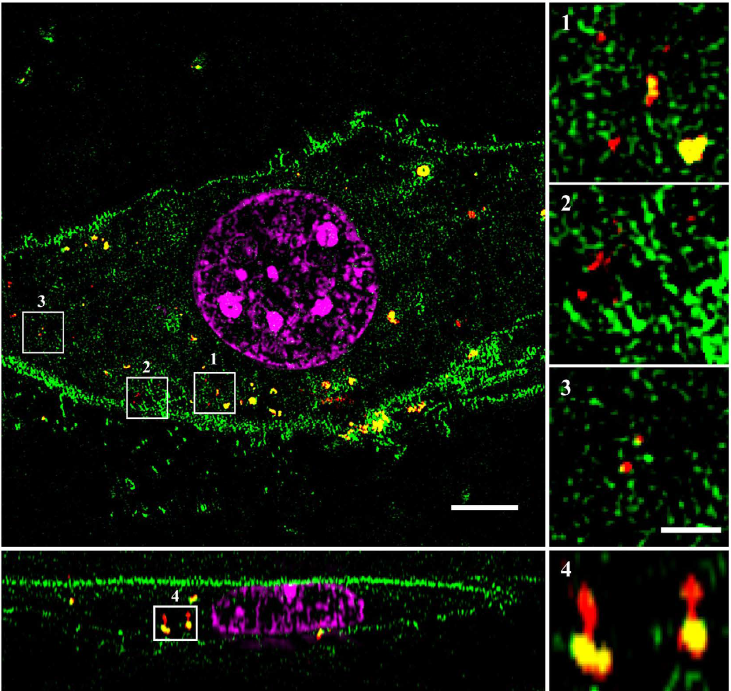
Green: mEGFP, red: Cy3 labeled MSNs,
magenta: DAPI counterstaining
Notably, a high payload of the spice curcumin encapsulated in CD-NP acts extremely rapidly on cell metabolism resulting in an immediate and complete inhibition of cell growth and in efficient cancer-cell killing only few hours after incubation. We discovered this early onset of anti-cancer action by live-cell time-lapse fluorescence microscopy using a high-throughput environmental stage.
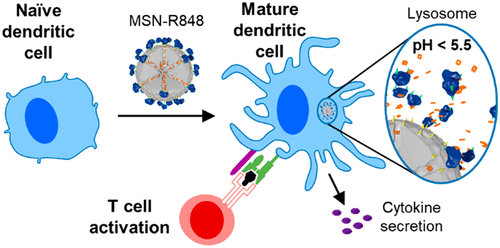
Present work in the group extends the functional scope of our diverse multifunctional mesoporous nanoparticles towards modulating the immune response of different types of macrophages. While many of the known immunomodulators tend to be hydrophobic and thereby are challenging candidates for medical administration, encapsulation in mesoporous carriers leads to rapid and efficient cellular uptake and intracellular release. It is of great interest to understand the impact of different carrier systems and their peripheral molecular functionalization on cellular uptake and immunomodulation response. Promising applications could involve treatment of autoimmune diseases and cancer.